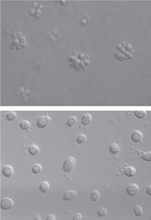
In F. Scott Fitzgerald’s short story, “The Curious Case of Benjamin Button,” an old man gets younger with each passing day, a fantastic concept recently brought to life on film by Brad Pitt. In a lab in Boston, a research team has used genetic engineering to accomplish something similarly curious, turning frail-looking mice into younger versions of themselves by stimulating the regeneration of certain tissues. The study helps explain why certain organs and tissues break down with age and, researchers say, offers hope that one day such age-related deterioration can be thwarted and even reversed.
As we age, many of our cells stop dividing. Our organs, no longer able to rejuvenate themselves, slowly fail. Scientists don’t fully understand what triggers this, but many researchers suspect the gradual shrinking of telomeres, the protective DNA caps on the end of chromosomes. A little bit of telomere is lost each time a cell divides, and telomerase, the enzyme that maintains caps, isn’t typically active in adult tissues. Another piece of evidence: People with longer telomeres tend to live longer, healthier lives, whereas those with shorter telomeres suffer more from age-related diseases, such as diabetes, Alzheimer’s, and heart disease.
Several years ago, Ronald DePinho, molecular biologist and director of the Belfer Institute of the Dana-Farber Cancer Institute, and colleagues at Harvard Medical School in Boston genetically engineered mice to lack a working copy of the telomerase gene. The animals died at about 6 months—that’s young for mice, which usually live until they are about 3 years old—and seemed to age prematurely. At an early age, their livers and spleens withered, their brains shrank, and they became infertile. By early adulthood, these mice exhibited many of the maladies seen in 80-year-old humans.
DePinho says he wondered what would happen to the aging process in these mice if they suddenly began making telomerase again. “Would [we] slow it, stabilize it, or would we reverse it?” He and his colleagues genetically engineered a new batch of mice with the same infirmity, but this time they added back a telomerase gene that became active only when the mice received a certain drug. The researchers kept the gene off during development and let these mice prematurely age, as the previous ones had. But then at 6 months, the team switched on the telomerase gene.
The burst of telomerase production spurred almost total recovery. The rodents became fertile, their livers and spleens increased in size, and new neurons appeared in their brains, the researchers reported online yesterday in Nature.
The ability to reverse age deterioration in the mutant mice indicates that the cells that divide to replenish tissues don’t simply die when their telomere clock expires, says DePinho. They apparently persist in a dormant state from which they can be revived. “One could imagine applying this approach to humans,” he says, focusing the therapy on specific tissue types such as the liver, where telomerase is thought to play an important role in regeneration after damage by hepatitis, parasitic infection, and alcoholism.
K. Lenhard Rudolph, who studies stem cell aging at the University of Ulm in Germany, says that the results are encouraging for people with diseases that cause accelerated aging, like progeria, because the mice in this study were rescued despite already suffering from the effects of chronic disease. “It is a proof of principle that telomeres are at work here.”
Drug companies and researchers are seeking ways to restore, protect, or extend a person’s telomeres, but the jury is still out on whether such interventions can slow the symptoms of aging, let alone reverse them. Telomere investigator Maria Blasco of the Spanish National Cancer Research Center in Madrid cautions that DePinho’s experiment shouldn’t raise people’s expectations of antiaging therapies just yet. “This study uses genetically modified mice,” she says. “What remains a very important question in the field is can you delay aging in a normal mouse?”
DePinho agrees with those concerns. He also warns that his approach has potential drawbacks, as increasing telomerase activity beyond its natural levels can cause cancer. Still, that may not be an insurmountable problem if telomerase levels can be carefully controlled. DePinho notes that the mice in his study, whose telomerase activity was returned to a natural level, didn’t develop tumors.
:: Read original here ::
 GUELPH, CANADA—When plucking a snail from the beach you’d be lucky to snag a left-coiling shell. That’s because only 5% of all snails are “lefties,” new research shows. Shell enthusiasts have long marveled at the lack of sinistral (left-coiling) snails among their collections, especially when other shelled mollusks, such as clams and the now-extinct ammonites—nautiluslike creatures that sported dozens of tentacles inside spiraled shells—are just as likely to be left- as right-coiling. Now, in the largest survey of its kind, researchers inspected more than 55,000 snail species—representing two-thirds of all gastropods—to reveal that left-coiling has arisen more than 100 times, and yet few of the species that have made the switch have been particularly successful. In the rare cases where left-coiling took off, it was almost always on land, the team reported here in a presentation last week at the annual meeting of the Canadian Society of Zoologists. The researchers don’t know why sinistrality is so rare underwater, but the most likely explanation, they say, is that unlike land snails that tend to hang around where they hatch out, the microscopic young of sea snails are carried on ocean currents that make the chance of meeting and reproducing with another left-coiling nest-mate slim. Without such a meeting, the left-coiling lineage goes extinct.
GUELPH, CANADA—When plucking a snail from the beach you’d be lucky to snag a left-coiling shell. That’s because only 5% of all snails are “lefties,” new research shows. Shell enthusiasts have long marveled at the lack of sinistral (left-coiling) snails among their collections, especially when other shelled mollusks, such as clams and the now-extinct ammonites—nautiluslike creatures that sported dozens of tentacles inside spiraled shells—are just as likely to be left- as right-coiling. Now, in the largest survey of its kind, researchers inspected more than 55,000 snail species—representing two-thirds of all gastropods—to reveal that left-coiling has arisen more than 100 times, and yet few of the species that have made the switch have been particularly successful. In the rare cases where left-coiling took off, it was almost always on land, the team reported here in a presentation last week at the annual meeting of the Canadian Society of Zoologists. The researchers don’t know why sinistrality is so rare underwater, but the most likely explanation, they say, is that unlike land snails that tend to hang around where they hatch out, the microscopic young of sea snails are carried on ocean currents that make the chance of meeting and reproducing with another left-coiling nest-mate slim. Without such a meeting, the left-coiling lineage goes extinct.