The daily smells of the world—the freshness of spring flowers, the smokiness of charred morning toast, the invigorating aroma of a cup of coffee—are lost on those with anosmia, a complete inability to detect odors. Now, thanks to a trio of people who feel no pain, a new study has pinpointed the first set of mutations that are to blame for this condition. The finding could help scientists better understand the gradual loss of smell that happens with age.
The discovery was somewhat happenchance. It began when neurobiologist Frank Zufall of Saarland University in Homburg, Germany, was contacted by pain researcher John Wood of University College London and geneticist Geoffrey Woods of the University of Cambridge in the United Kingdom. Wood and Woods had been studying three people with an inability to feel pain. In 2006, they found that all had mutations in a gene that codes for a sodium channel called Nav1.7 that is involved in the firing of neurons. The researchers also noticed that the volunteers reported having no sense of smell. When the duo created mice with similar mutations, the pups were free of pain, but they also had difficulty smelling; they seemed unable to pick up the odor of their mother’s teats to suckle, for example.
To investigate how the lack of these channels causes anosmia, Zufall and his team studied the genetically engineered mice. They discovered that although neurons in the rodents’ noses respond to different odors, they were unable to transmit these signals to the olfactory bulb, the region at the front of the brain that processes smell.
The researchers also confirmed that the mice couldn’t smell. In a series of experiments, the rodents didn’t avoid the smell of predators, nor did they retrieve their pups when they were scattered around their cage, the researchers report online today in Nature. “This behavior supports that fact that the mice aren’t smelling,” says Lisa Stowers, a neurobiologist at The Scripps Research Institute in San Diego, California, who studies olfaction.
Zufall hopes to turn up more genes underlying anosmia by encouraging doctors to carry out a smell test in patients who present with neurological problems. Peter Mombaerts, an olfaction neuroscientist at the Max Planck Institute of Biophysics in Frankfurt, Germany, agrees that this would be a good approach. “It is a very cheap test,” he says, but he cautions that few doctors currently do it, even though smell can be an important indicator of neurological disease. For example, in Alzheimer’s patients, smell is often one of the first things to deteriorate.
The number of people that are anosmic from birth is likely quite small, says Mombaerts, but by better understanding how olfactory signals are disrupted in people born with this condition, researchers hope to gain insight into why many more develop it later in life.
:: Read original here ::
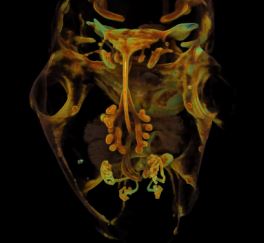



 Moms, want your children to eat their greens? Then you have to eat them, too, at least while you’re pregnant. Researchers have found that offspring of mouse mothers fed a diet enhanced with cherry and mint flavors during pregnancy continued to prefer these flavors into adulthood, while mice from mothers fed on a bland diet had no food preference. The rodents with a penchant for mint-cherry food developed larger glomeruli, the region of the brain responsible for processing odor—the first evidence that exposure to odors in the womb alters the way the brain develops. From the fetus’ point of view, this is a good evolutionary strategy; eat the foods that your mother ate because they are probably safe. It is likely that all mammals, including humans, develop their sense of taste in this same way, the researchers report online today in the Proceedings of the Royal Society B, so expectant moms, be careful the next time you have a hankering for anchovies with chocolate sauce.
Moms, want your children to eat their greens? Then you have to eat them, too, at least while you’re pregnant. Researchers have found that offspring of mouse mothers fed a diet enhanced with cherry and mint flavors during pregnancy continued to prefer these flavors into adulthood, while mice from mothers fed on a bland diet had no food preference. The rodents with a penchant for mint-cherry food developed larger glomeruli, the region of the brain responsible for processing odor—the first evidence that exposure to odors in the womb alters the way the brain develops. From the fetus’ point of view, this is a good evolutionary strategy; eat the foods that your mother ate because they are probably safe. It is likely that all mammals, including humans, develop their sense of taste in this same way, the researchers report online today in the Proceedings of the Royal Society B, so expectant moms, be careful the next time you have a hankering for anchovies with chocolate sauce.